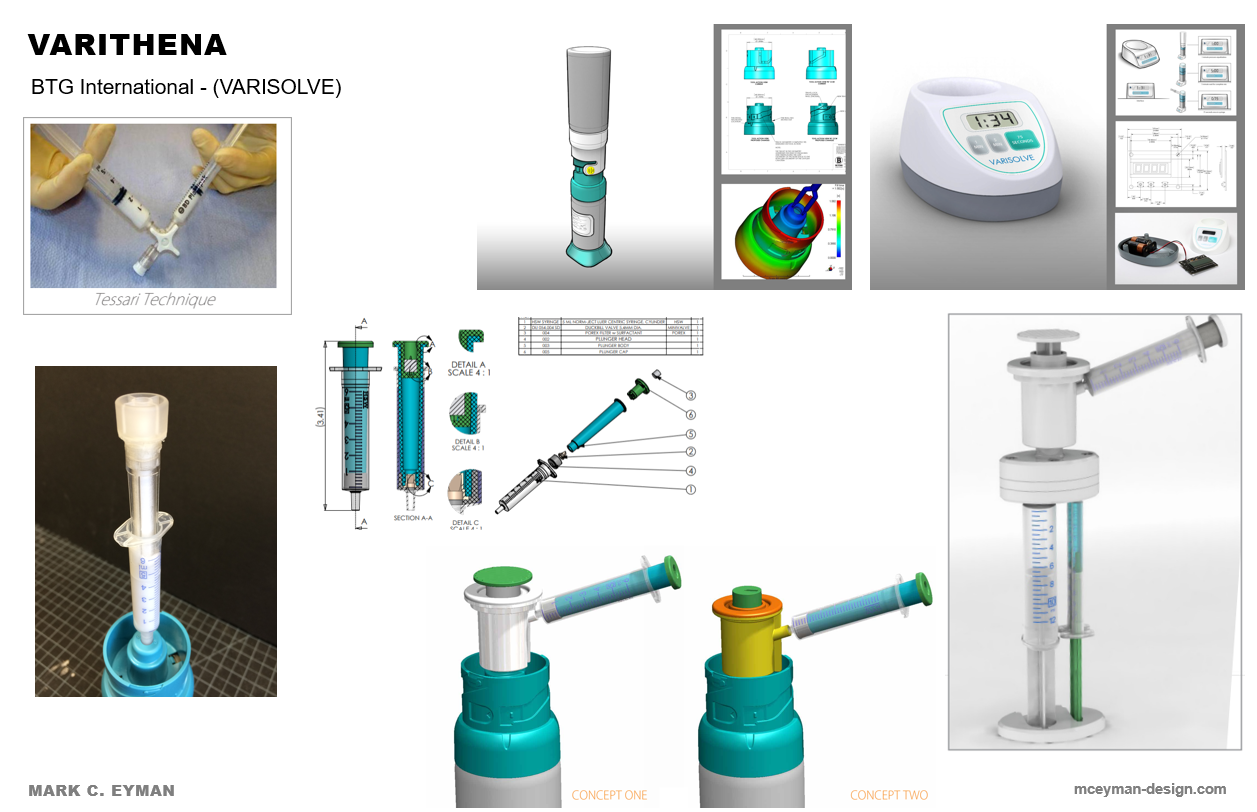
This product is now called Varithena owned by Boston Scientific. Description:
Varithena® (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena® improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
I worked as a consultant at Beyond Design in Chicago working with John DeFoggie, the Managing Partner of BPTM, LLC who brought the project to us for BTG International (sold to Boston Scientific in 2019). Our task was to improve the product function as it was when previously submitted to the FDA and failed, and also to create several contingency technologies to address concerns. In the end, the product was approved with some minor improvements to the existing design. (video of function)
My role in the development:
Concept, Design and Prototype improvements to the current injection molded parts
Communicate mold design changes using SolidWorks CAD data and presentations to the molder in Ireland
Research, evaluate, concept, prototype, validate micro foam with the polidocanol solution and document any concepts that may be viable alternatives to the current design
Work with a former Baxter Engineer on contingency technologies and accompany him to factory audits
Research, evaluate, create concepts and prototype for validation any technology which could also accomplish the micro-foam
Maintain the Design History File for BTG (based in England)
Note: We did outsource Mold flow analysis to the molder, and tolerance stack up studies to and outside Engineering firm. I managed the internal and external prototyping and conducted the evaluations.